WE ARE TRANSFORMING THE DELIVERY OF NITRIC OXIDE
We are harnessing the power of nitric oxide for wound healing and the treatment of skin and soft tissue infections and inflammation.
harnessing the power of nitric oxide
In the human body, nitric oxide (NO) is a naturally-occurring signaling molecule that is known to regulate blood flow (1,9-11), modulate inflammation (1-4), stimulate tissue regeneration (3,9), and combat infection (4-8). As a result, NO has huge promise as a powerful weapon in the battle against multiple diseases.
Our innovative technology platform, IonoJet™, harnesses the power of NO for wound healing and the treatment of skin and soft tissue infections and inflammation. The IonoJet™ is a [high-energy] plasma device that generates NO in the form of a plasma stream allowing for targeted delivery of NO to the specific tissues requiring treatment.
The mission of Origin is to leverage our patented IonoJet™ technology in the development of therapies based on the convenient, affordable and non-invasive delivery of NO directly to the treatment site.
CAUTION – The IonoJet™ is an Investigational Device limited by Federal (or United States) Law to investigational use.
THE SCIENCE
The medical use of high-energy plasma is an innovative and emerging field combining physics, life sciences and clinical medicine to use plasmas for therapeutic applications.
OUR TECHNOLOGY
Our technology, IonoJet™, is based on Nobel prize-winning research (13,14) and is designed to surpass current standards of care in efficacy, side effects, and/or cost-effectiveness across a wide range of indications.
OUR TARGET INDICATIONS
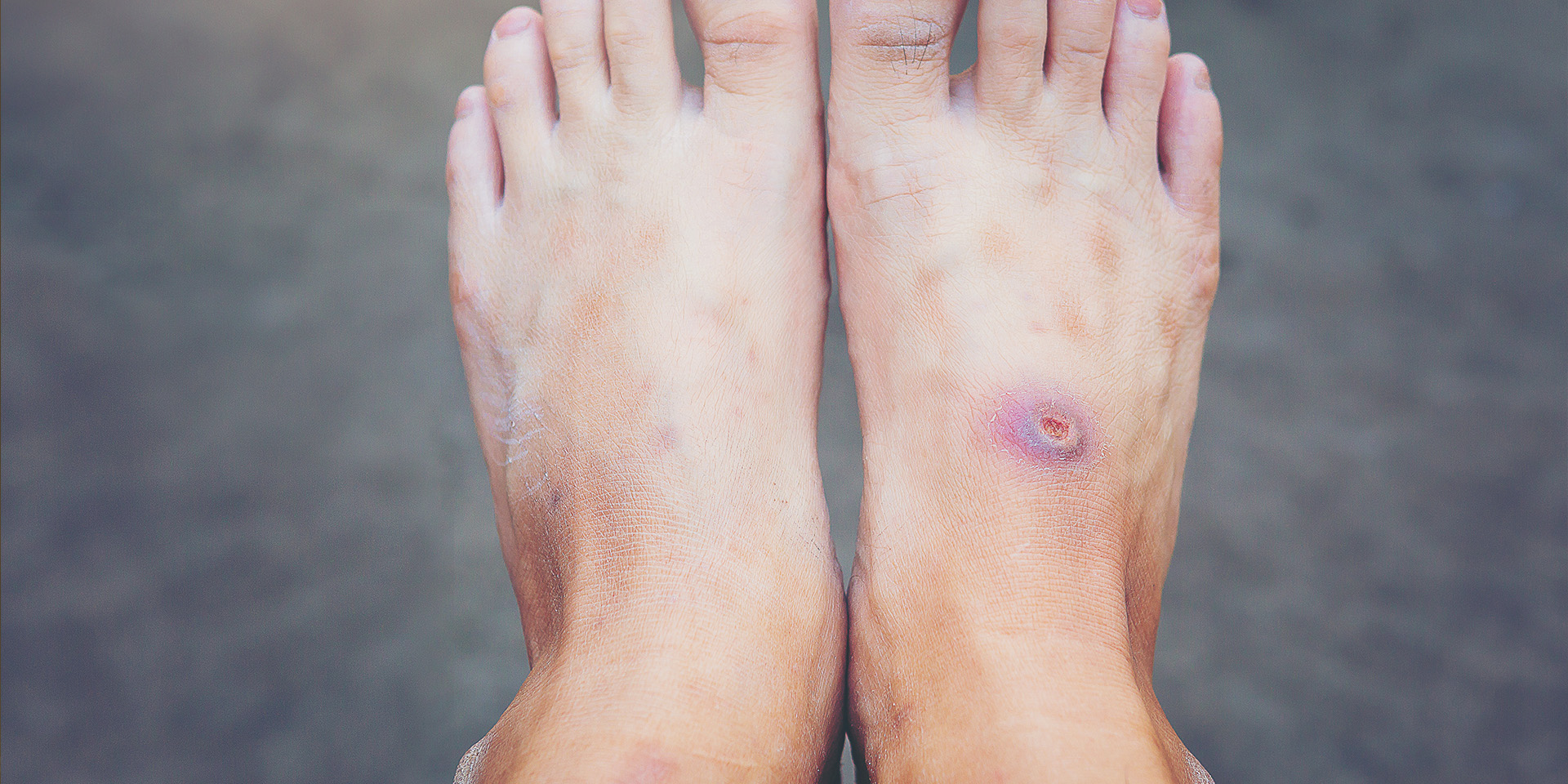
Our technology platform has the potential to be used for multiple therapeutic purposes including as an anti-infective, anti-inflammatory and tissue-regenerative therapy for chronic wounds and skin and soft tissue disorders.
The Rationale for Nitric Oxide Therapies
Despite the remarkable advances in medicine in the past century, there are still diseases that remain frustratingly beyond the grasp of the latest drugs, devices and surgical interventions. These are not niche conditions, but issues affecting billions of people worldwide including the global diabetic epidemic, chronic inflammatory diseases, and antimicrobial resistance.
NO functions as an important signaling molecule, acting as a messenger molecule, transmitting signals to cells in the cardiovascular, nervous and immune systems. Due to its chemical composition, NO is much more reactive than other signaling molecules and its small size allows it to diffuse through cell membranes and walls to perform various signaling functions in the human body systems.
The main site of the molecule’s synthesis is the inner layer of blood vessels, where it diffuses to underlying smooth muscle cells and causes them to relax. This relaxation causes the walls of blood vessels to dilate, which in turn increases blood flow through the vessels and decreases blood pressure. NO’s role in dilating blood vessels makes it an important controller of blood pressure. NO is also produced by neurons and is used by the nervous system as a neurotransmitter to regulate various bodily functions including digestion, blood flow, memory and vision. In the immune system, NO is produced by macrophages, which are a type of white blood cell that engulfs bacteria and other foreign particles that have invaded the body. The NO released by macrophages kills bacteria, other parasites, and tumor cells by disrupting their metabolism.
NO is critical in defending against infection. Depending on its concentration, NO exerts antimicrobial effects in two ways. At low concentrations, NO acts as a signaling molecule that promotes the growth and activity of immune cells. At high concentrations, NO covalently binds DNA, proteins and lipids, thereby inhibiting or killing target pathogens. NO is an integral and highly conserved part of the host immune response. Few bacteria are able to escape the antimicrobial effect of NO.
NO is a key molecule in dermal wound healing and tissue regeneration. There are many reports that state NO represents a promising method to promote wound healing by enhancing cell proliferation, collagen deposition, and angiogenic activities improving granulation tissue formation.
Our Ionojet™ technology has potential application across a broad spectrum of disease states including wound healing, combatting infection, dermal therapeutics, musculoskeletal conditions, dental infections and respiratory tract infections.