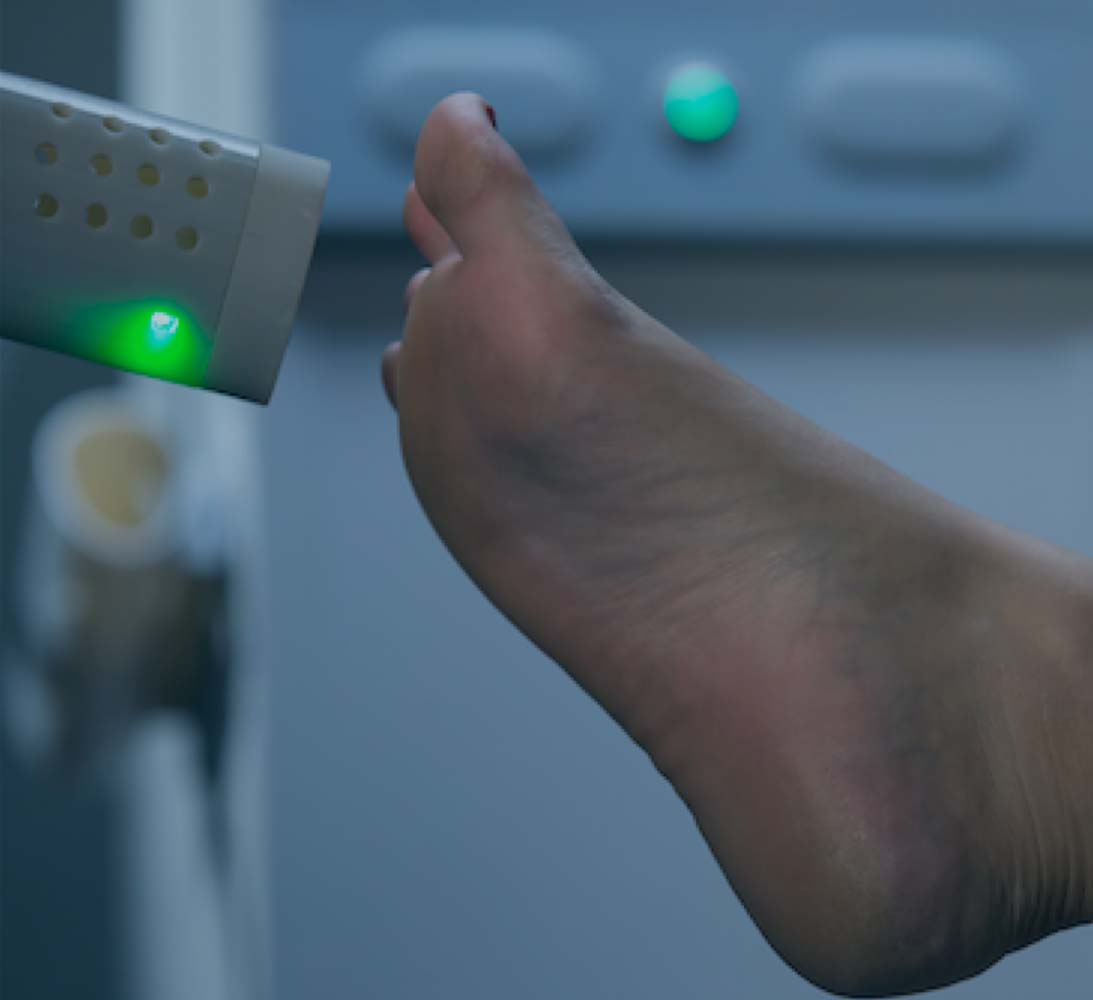
TECHNOLOGY PLATFORM
TARGETED DELIVERY OF NITRIC OXIDE IN THE CLINIC
Origin’s mission is to leverage our patented IonoJet™ technology in the development of therapies based on the convenient, affordable and non-invasive delivery of nitric oxide (NO) directly to the treatment site. Our goal is to become the leading provider of topical NO treatments using our proprietary IonoJet™ device for various therapeutic purposes, including as an anti-infective, anti-inflammatory and tissue-regenerative therapy for chronic wounds and skin and soft tissue disorders.
The plasma/NO stream generated by our device has the potential to promote healing in various ways as a result of the effect that NO has on immune system regulation, blood vessel regulation and tissue regeneration and in defending against infection. In particular, NO is a potential wound therapeutic agent due to its ability to regulate inflammation, increase blood flow, eradicate bacterial infections, and promote the growth and activity of immune cells.
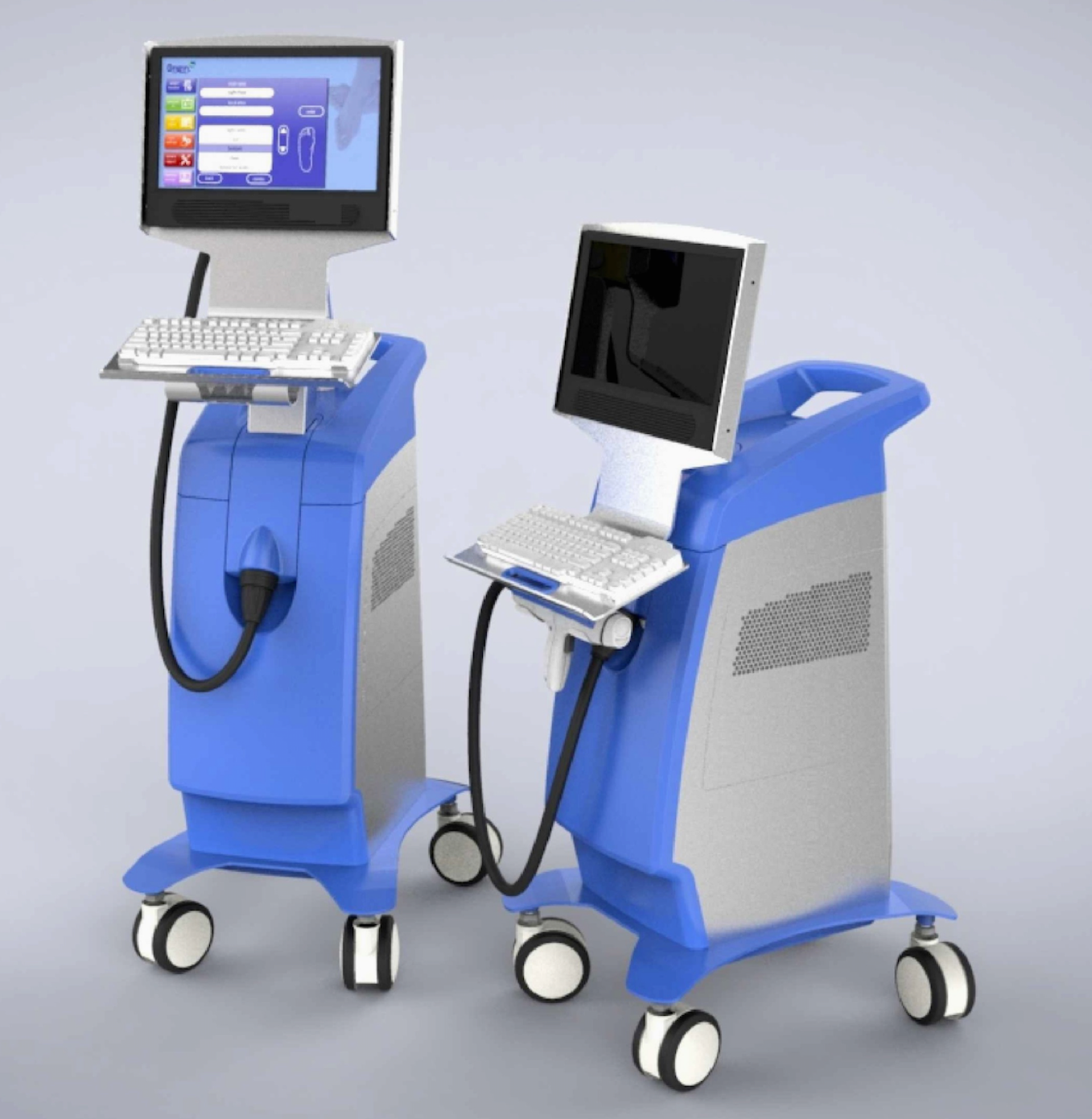
This device is currently under development. The prototype shown here is the U.S. version of our therapy delivery platform that was used in our dose-ranging clinical trial and in our proof-of-concept studies. Currently the device is in the process of being modified for use in our planned pivotal trial.
POWERFUL TECHNOLOGY PLATFORM
Our IonoJet™ technology platform turns atmospheric air into a plasma/NO stream that has been shown in investigations to date: (i) to be non-toxic, (ii) to generate NO activity up to 3 cm below the skin, and (iii) to stimulate sustained biological activity in tissue for up to an hour after delivery of the therapy.
Our patented Arc Discharge Technologies enable the production of NO up to 1,000ppm (as compared with other technologies producing orders of magnitude less), using only the nitrogen and oxygen present in ambient air. The reaction requires temperatures of around 4,000ºC (45).
The IonoJet™ system employs an array of super-cooling technologies and safety control systems to enable therapeutic application at temperatures in the range of 45ºC, while maintaining NO levels up to 1,000ppm (45).
Based upon clinician feedback and the results of our feasibility trial, we were able to identify several desirable changes that we believe will enhance commercial adoption of the Ionojet™, and we have been working on the reengineered design of our device in our own facility. We have made what we believe are significant improvements to our Ionojet™ technology, all of which we are seeking to protect with new U.S. and international patent filings.
CLINICAL STUDIES
To date, our technology has been studied in (i) animal safety studies that were completed between 2013 and 2016; (ii) a single-center study of 40 patients in 2013 to treat chronic wounds; (iii) a clinical pilot study in 10 patients with diabetic foot ulcers completed in 2016; and (iv) a clinical dose-ranging feasibility trial that was commenced in 2017 and completed in 2018 for the treatment of patients with diabetic foot ulcers (GENESIS).
CAUTION – The IonoJet™ is an Investigational Device limited by Federal (or United States) Law to investigational use.